net dipole moment|More : Clark A moment is a measure of a turning force about an axis. When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning .
Resultado da You’ve got (a lot) of options. Designer handbags, purses, crossbody bags, tote bags, backpacks, travel bags , shoulder bags & hobos, satchels, clutches, belt bags.you get the idea. We’ve got women’s .
0 · net dipole moment of nh3
1 · net dipole moment of ch2br2
2 · net dipole moment meaning
3 · net dipole moment formula
4 · net dipole moment calculator
5 · how do dipoles cancel out
6 · dipole moment is shown by
7 · calculate dipole moment
8 · More
WEB88.7k 100% 1min 32sec - 1080p. Safada do rabão. 20.2k 87% 2min - 720p. Guardando minha pica no cuzão da gordinha. 216.9k 99% 2min - 720p. Gordinha gostosa. 20.1k .
net dipole moment*******Dipole moments occur when there is a separation of charge. They can occur between two ions in an ionic bond or between atoms in a covalent bond; dipole moments arise from differences in electronegativity. The larger the difference in electronegativity, .
The net dipole is the measurable, which is called the dipole moment. Dipole moment is equal to the product of the partial charge and the distance. The equation for dipole .
Learn what dipole moment is, how to calculate it and how it relates to polarity and electronegativity. See examples of dipole .
Learn how to assess the polarity of a molecule based on its bonding and structure, and how to calculate the dipole moment using electronegativity an.Due to their different three-dimensional structures, some molecules with polar bonds have a net dipole moment (HCl, CH 2 O, NH 3, and CHCl 3), indicated in red, whereas others . A moment is a measure of a turning force about an axis. When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning .
Learn what a dipole moment is, how to calculate it, and how it relates to molecular polarity and geometry. See examples of dipole moments in CO2, H2O and NH3 molecules.
net dipole moment MoreLearn about the types and properties of molecules, such as polar and nonpolar covalent bonds, dipole moments, and intermolecular forces. This web page is part of a free . This organic chemistry video explains how to determine if a molecule is polar and has net dipole moment. The difference in electronegativity can be used to .
Learn how to calculate and represent bond dipole moments and molecular polarity using electronegativity differences and vector addition. Explore the properties and examples of polar and nonpolar .
net dipole moment In chemistry, dipole moments are applied to the distribution of electrons between two bonded atoms. The existence of a dipole moment is the difference between polar and nonpolar bonds. Molecules with a net dipole moment are polar molecules. If the net dipole moment is zero or very, very small, the bond and molecule are considered to .
Remember, molecular dipole moments are just the vector sum of all of the dipole moments of the individual bonds, and the dipole moments are all . to cancel this large one out. In fact, .
Dipole Moment. When two electrical charges, of opposite sign and equal magnitude, are separated by a distance, an electric dipole is established. The size of a dipole is measured by its dipole moment (\(\mu\)). Dip ole moment is measured in Debye units, which is equal to the distance between the charges multiplied by the charge (1 Debye eq uals .
The definition of a dipole moment is the measurement of the overall (or net) polarity in a molecule. The polarity is a measure of the positive and negative charges in the molecule and how they .
The electric dipole moment is a measure of the separation of positive and negative electrical charges within a system: that is, a measure of the system's overall polarity.The SI unit for electric dipole moment is the coulomb-meter (C⋅m). The debye (D) is another unit of measurement used in atomic physics and chemistry.. Theoretically, an electric dipole .
A dipole moment is the turning force a fixed charge has on a dipole in a molecule. . If you use a molymod set, you will kind of see that your net dipole moment would be directed upward in this case. And so the individual bond dipoles are going to add to give you a molecular dipole, .When you place a molecule with an electric dipole in an electric field, a force acts to turn the molecule so that the positive and negative ends line up with the field. The magnitude of the turning force is given by the formula. µ = q × d. where q is the amount of charge and d is the distance between the two charges. µ is the turning moment.
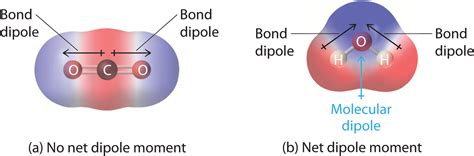
This organic chemistry video explains how to determine if a molecule is polar and has net dipole moment. The difference in electronegativity can be used to .Using the equation above, the dipole moment is calculated to be 1.85 D by multiplying the distance between the oxygen and hydrogen atoms by the charge difference between them and then finding the components of each that point in the direction of the net dipole moment (the angle of the molecule is 104.5˚).The dipole moment of a molecule is therefore the vector sum of the dipole moments of the individual bonds in the molecule. If the individual bond dipole moments cancel one another, there is no net dipole moment. Such is the case for CO 2, a linear molecule (part (a) in Figure 2.2.8). Each C–O bond in CO 2 is polar, yet experiments show that .
Individual bond dipole moments are indicated in red. Due to their different three-dimensional structures, some molecules with polar bonds have a net dipole moment (HCl, CH 2 O, NH 3, and CHCl 3), indicated in purple, whereas others do not because the bond dipole moments cancel (BCl 3, CCl 4, PF 5, and SF 6).

Dipole Moment. When two electrical charges, of opposite sign and equal magnitude, are separated by a distance, an electric dipole is established. The size of a dipole is measured by its dipole moment (\(\mu\)). Dip ole moment is measured in Debye units, which is equal to the distance between the charges multiplied by the charge (1 Debye eq uals .(a) The net force on the dipole is zero, but the net torque is not. As a result, the dipole rotates, becoming aligned with the external field. (b) The dipole moment is a convenient way to characterize this effect. The d → d → points in the same direction as p → p →.
水分子是一種極性化合物。這是因為其電子的不均勻分佈成鈍角狀結構。 此圖顯示出電荷的分離現象,負電荷占有紅色區域,正電荷占有藍色區域。 以有限距離隔開的兩個同電量的異性電荷所形成的物理電偶極子與其電場線。 任意點偶極子(電偶極子、磁偶極子、聲偶極子等等)的場線。The dipole moment measures the extent of net charge separation in the molecule as a whole. In diatomic molecules, the bond dipole moment determines the molecular polarity. When a molecule contains more than one bond, the geometry must be taken into account. If the bonds in a molecule are arranged such that the vector sum of their bond moments .The dipole moment is defined as the product of the partial charge Q on the bonded atoms and the distance r between the partial charges: μ = Qr. where Q is measured in coulombs (C) and r in meters. The unit for dipole moments is the debye (D): 1 .The dipole moment of a molecule and its overall polarity depends on the magnitude and direction of individual polar bonds and their dipole moments.. Remember, for molecules with one polar bond, the molecular dipole is determined simply based on the dipole moment of that bond: Now, if there are multiple polar bonds, the molecular dipole .
More知乎专栏 - 随心写作,自由表达 - 知乎
Resultado da Antes de te enviarmos, você deve fazer o log in.
net dipole moment|More